San Diego, March 13, 2023 – Viracta Therapeutics, Inc. (Nasdaq: VIRX), a precision oncology company focused on the treatment and prevention of virus-associated cancers that impact patients worldwide, today announced financial results for the fourth quarter and full year 2022 and provided an update on recent corporate developments.
“Over the past months we made important progress towards our goal of developing Nana-val as a tumor agnostic therapy for patients with difficult-to-treat EBV-associated cancers,” said Mark Rothera, President and Chief Executive Officer of Viracta. “The latest data from our solid tumor trial showed a confirmed partial response and no dose limiting toxicities through three of five planned dose levels in patients with recurrent or metastatic nasopharyngeal carcinoma. We look forward to reporting data from the full dose escalation part of the trial, selecting our recommended Phase 2 dose, initiating the Phase 2 nasopharyngeal carcinoma expansion cohort and evaluating Nana-val in additional EBV-positive solid tumors later this year. Our pivotal NAVAL-1 trial site footprint continues to grow as we seek to advance multiple EBV-positive lymphoma subtypes into Stage 2 of this global study. With these anticipated milestones ahead of us and a cash runway into late 2024, we believe Viracta is well-positioned to generate value for patients and shareholders over the coming months.”
Program Highlights and Anticipated Milestones
Pivotal NAVAL-1 trial of Nana-val in relapsed/refractory (R/R) EBV+ lymphoma
Phase 1b/2 trial of Nana-val in patients with recurrent/metastatic (R/M) EBV+ nasopharyngeal carcinoma (NPC) and other advanced EBV+ solid tumors
Regulatory
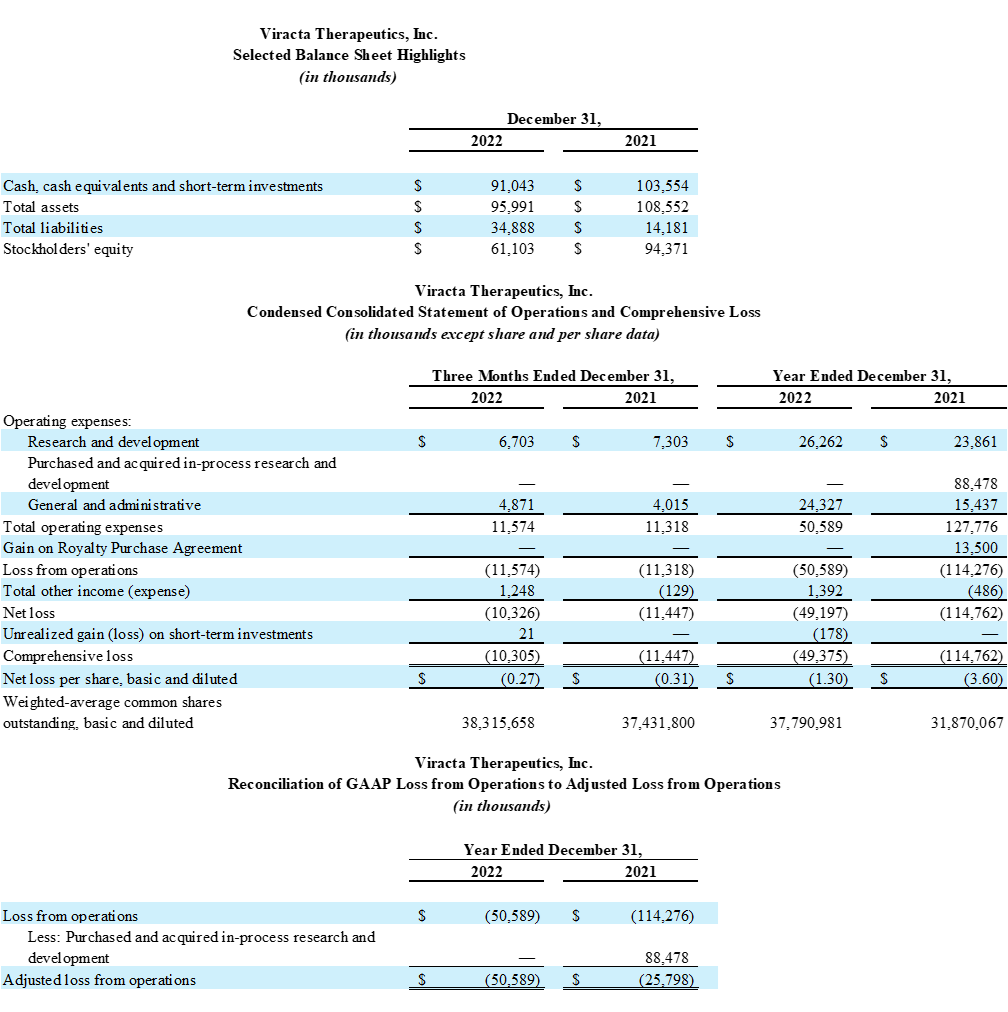
Fourth Quarter and Full Year 2022 Financial Results
About NAVAL-1
NAVAL-1 (NCT05011058) is a global, multicenter trial of Nana-val in R/R EBV+ lymphoma. The trial employs a Simon two-stage design where participants are enrolled into six indication cohorts based on EBV+ lymphoma subtype in Stage 1. If a pre-specified activity threshold is reached within a lymphoma subtype in Stage 1 (n=10), additional patients will be enrolled in Stage 2 for a total of 21 patients. EBV+ lymphoma subtypes demonstrating promising activity in Stage 2 may be further expanded following discussion with regulators to potentially support registration.
About the Phase 1b/2 Trial of Nana-val in R/M EBV+ NPC and Other EBV+ Solid Tumors
This Phase 1b/2 trial (NCT05166577) is an open-label, multinational trial evaluating Nana-val alone and in combination with pembrolizumab. The Phase 1b dose escalation part is designed to evaluate safety and to determine the RP2D of Nana-val in patients with R/M EBV+ NPC. In Phase 2, up to sixty patients with R/M EBV+ NPC will be randomized to receive Nana-val at the RP2D with or without pembrolizumab to evaluate safety, overall response rate, and potential pharmacodynamic markers. Additionally, patients with other advanced EBV+ solid tumors will be enrolled to receive Nana-val at the RP2D in a Phase 1b dose expansion cohort.
About Nana-val (Nanatinostat and Valganciclovir)
Nanatinostat is an orally available histone deacetylase (HDAC) inhibitor being developed by Viracta. Nanatinostat is selective for specific isoforms of Class I HDACs, which are key to inducing viral genes that are epigenetically silenced in Epstein-Barr virus (EBV)-associated malignancies. Nanatinostat is currently being investigated in combination with the antiviral agent valganciclovir as an all-oral combination therapy, Nana-val, in various subtypes of EBV-associated malignancies. Ongoing trials include a pivotal, global, multicenter, open-label Phase 2 basket trial in multiple subtypes of relapsed/refractory EBV+ lymphoma (NAVAL-1) as well as a multinational Phase 1b/2 trial in patients with EBV+ recurrent or metastatic nasopharyngeal carcinoma and other EBV+ solid tumors.
About Viracta Therapeutics, Inc.
Viracta is a precision oncology company focused on the treatment and prevention of virus-associated cancers that impact patients worldwide. Viracta’s lead product candidate is an all-oral combination therapy of its proprietary investigational drug, nanatinostat, and the antiviral agent valganciclovir (collectively referred to as Nana-val). Nana-val is currently being evaluated in multiple ongoing clinical trials, including a pivotal, global, multicenter, open-label Phase 2 basket trial for the treatment of multiple subtypes of relapsed/refractory Epstein-Barr virus-positive (EBV+) lymphoma (NAVAL-1), as well as a multinational, open-label Phase 1b/2 trial for the treatment of EBV+ recurrent or metastatic nasopharyngeal carcinoma and other EBV+ solid tumors. Viracta is also pursuing the application of its “Kick and Kill” approach in other virus-related cancers.
For additional information please visit www.viracta.com.
Forward Looking Statements
This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, without limitation, statements regarding: the details, timeline and expected progress for Viracta's ongoing and anticipated trials and updates regarding the same, including NAVAL- 1 and the Phase 1b/2 trial of Nana-val in EBV+ solid tumor and the sufficiency of current cash reserves to fund ongoing operations for the specified period. Risks and uncertainties related to Viracta that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to: Viracta's ability to successfully enroll patients in and complete its ongoing and planned clinical trials; Viracta's plans to develop and commercialize its product candidates, including all oral combinations of nanatinostat and valganciclovir; the timing of initiation of Viracta's planned clinical trials; the timing of the availability of data from Viracta's clinical trials; previous preclinical and clinical results may not be predictive of future clinical results; the timing of any planned investigational new drug application or new drug application; Viracta's plans to research, develop and commercialize its current and future product candidates; the clinical utility, potential benefits and market acceptance of Viracta's product candidates; Viracta's ability to manufacture or supplying nanatinostat, valganciclovir and pembrolizumab for clinical testing; Viracta's ability to identify additional products or product candidates with significant commercial potential; developments and projections relating to Viracta's competitors and its industry; the impact of government laws and regulations; Viracta's ability to protect its intellectual property position; and Viracta's estimates regarding future expenses, capital requirements and need for additional financing in the future.
These risks and uncertainties may be amplified by the COVID-19 pandemic, which has caused significant economic uncertainty. If any of these risks materialize or underlying assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption "Risk Factors" and elsewhere in Viracta's reports and other documents that Viracta has filed, or will file, with the SEC from time to time and available at www.sec.gov.
The forward-looking statements included in this communication are made only as of the date hereof. Viracta assumes no obligation and does not intend to update these forward-looking statements, except as required by law or applicable regulation.
Financial tables attached
Investor Relations Contact:
Ashleigh Barreto
Head of Investor Relations & Corporate Communications
Viracta Therapeutics, Inc.
abarreto@viracta.com
SOURCE Viracta Therapeutics, Inc.